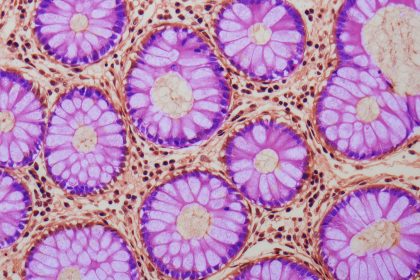
The administration of fecal matter from younger murine subjects to older counterparts has elucidated the profound influence microorganisms exert on the functional capacity of intestinal stem cells.
Following a fecal microbiota transplantation originating from younger mice, a specific facet of age-related intestinal deterioration in older subjects was successfully ameliorated. This reversal was propelled by an enhanced activation of intestinal stem cells, which are integral to the preservation of the intestinal lining.
These findings posit that such transplantations could potentially evolve into a therapeutic modality for age-associated intestinal ailments, including but not limited to inflammation and obesity.
“As organisms mature, the continuous regeneration of intestinal tissue decelerates, thereby augmenting susceptibility to gastrointestinal disorders,” states molecular biologist Hartmut Geiger, affiliated with Ulm University in Germany. “Our discoveries indicate that a more youthful microbial ecosystem can stimulate intestinal repair and foster functionality akin to that of younger intestines in aged subjects.”
Intestinal stem cells are indispensable for the maintenance of a robust and balanced gut environment. They are the biological drivers responsible for the constant renewal and replenishment of the intestinal epithelium, thereby ensuring sustained gastrointestinal operations.
Nevertheless, with advancing age, the pace of this regenerative process diminishes, leading to increased vulnerability to age-related dysfunctions of the gut.
In prior scientific investigations, Geiger, in collaboration with cell biologists Yi Zheng and Kodandaramireddy Nalapareddy from Cincinnati Children’s Hospital Medical Center, established a direct correlation between this attenuated regeneration and a compromised functional state of intestinal stem cells.
Furthermore, it is recognized that the microbial communities resident within our digestive tracts undergo alterations with age, and such shifts have been implicated in the pathogenesis of conditions like Parkinson’s disease, Alzheimer’s disease, and even visual impairments. The researchers sought to ascertain whether the gut microbiome also exerts an influence on stem cell activity.
Consequently, additional team members were enlisted, and a straightforward experimental paradigm was devised to evaluate this hypothesis: the transplantation of fecal samples between and within cohorts of aged and juvenile mice.
Upon the conclusion of the transplantation procedures, the researchers conducted detailed analyses of the intestinal tissues to identify any resultant modifications.
In the older mice, the observed impact was quite significant. There was a marked increase in stem cell activity, alongside an amplification of Wnt signaling, which is essential for the operational efficiency of these cells. The regeneration of the epithelium accelerated, and crucially, the gut demonstrated enhanced recovery capabilities following radiation-induced damage.
“This diminished signaling contributes to a reduction in the regenerative capacity of aged intestinal stem cells,” observes Zheng. “However, when the aged microbiota were supplanted by a younger microbial composition, the stem cells resumed the production of new intestinal tissue, mimicking the behavior of younger cells. This further underscores the profound impact of the symbiotic organisms inhabiting our bodies on human well-being.”
In the younger mice, the alteration was less pronounced. A merely slight decrement in stem cell activity, Wnt signaling, and regeneration was noted; the intestines continued to perform adequately. This suggests that aging intestines exhibit a considerably greater sensitivity to microbiome fluctuations compared to their younger counterparts.
An additional noteworthy revelation was that a contributing factor to the suppression of stem cell function in the aging gut is Akkermansia, a bacterium typically regarded as beneficial through various mechanisms, with evidence suggesting its potential to mitigate diet-induced obesity and depression-like behaviors in murine models.
Within aging mice, elevated concentrations of Akkermansia were found to actively impede Wnt signaling – a finding that implies the nature of gut bacteria is not inherently positive or negative, but rather their contribution is context-dependent.
This research does not represent a definitive breakthrough for human health; our biological systems (and intestines) are substantially more intricate than those of mice, necessitating distinct investigations to determine if this phenomenon is replicated in our species.
Nevertheless, the study does illuminate a promising avenue for future scientific exploration.
It also indicates that the decline in stem cell function associated with aging may not be an immutable process. By leveraging the capacity of gut microbes to modulate the regenerative mechanisms of intestinal tissue, scientists may eventually devise strategies to preserve intestinal health throughout the aging process.
This research has been formally disseminated in Stem Cell Reports.