Research spearheaded by academicians at Italy’s University of Camerino has revealed that the fatty sheaths safeguarding nerve cells can sustain damage from insufficient sleep, thereby impairing cognitive functions.
Through a series of diverse experimental procedures and analyses, the investigative cohort pinpointed chemical aberrations within oligodendrocyte cells as the root cause of this cellular detriment. These specialized cells are instrumental in managing the cholesterol essential for constructing the protective layers known as myelin.
“This investigation establishes oligodendrocytes as pivotal agents by establishing a correlation between sleep deprivation and compromised myelin integrity, diminished nerve signal transmission, and observable behavioral impairments,” is how the researchers articulated their findings in their published academic treatise.
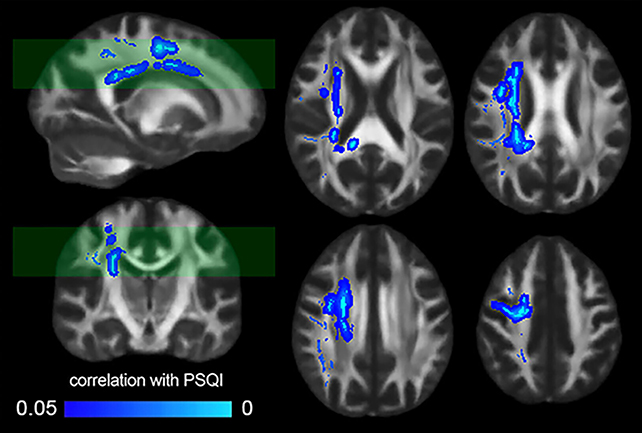
The scientific cadre meticulously examined MRI scans from a cohort of 185 healthy individuals, thereby corroborating the outcomes of prior investigations which had indicated a decline in the structural robustness of the brain’s white matter tracts coinciding with self-reported deteriorations in sleep quality.
Subsequently, the research team conducted empirical trials on rodents subjected to a stringent sleep deprivation regimen for a duration of ten days. While the physical dimensions of the neural fibers within the brains of these animals remained unaltered, the myelin sheath enveloping each neuron’s elongated projection, referred to as an axon, exhibited a reduced thickness when contrasted with a control group.
Additional exhaustive experimentation revealed that the communication rate between specific cerebral regions was approximately reduced by one-third in the rodents that had been deprived of sleep. Furthermore, this sleep deprivation resulted in a decrement in the synchronized activity across various brain areas.
Axons are fundamental conduits for neural communication; a deficit in sleep precipitates a thinning of the myelin sheath, which in turn decelerates neural signal propagation and elevates the susceptibility to mental exhaustion and cognitive cloudiness, phenomena evidenced by performance decrements in memory and motor function assessments conducted on the experimental rodents.
A comprehensive genetic profiling of the mice indicated that oligodendrocytes were no longer processing cholesterol with their customary efficiency.
“Our findings illuminate a potential role for dysregulation of oligodendrocyte cholesterol metabolism in the behavioral deficits associated with sleep deprivation and unveil a novel avenue for therapeutic intervention,” the investigators stated in their publication.
The scientific inquiry did not conclude there; the researchers administered the pharmaceutical agent cyclodextrin to the sleep-deprived rodents. This intervention facilitated a restoration of cholesterol transport, subsequently leading to discernible enhancements in both motor skills and memory recall, thereby substantiating their hypotheses.
It is imperative to acknowledge that the majority of this research employed animal models, necessitating future validation through human studies. Notwithstanding this acknowledged limitation, the findings provide compelling evidence of an intriguing mechanism by which insufficient sleep contributes to a general state of lethargy.
Looking forward, the insights gleaned from this study could potentially inform the development of therapeutic strategies aimed at mitigating some of the detrimental effects of chronic sleep deprivation, a condition implicated in a spectrum of adverse health outcomes.
“The escalating incidence of sleep deprivation constitutes a significant public health challenge in our contemporary society,” the researchers noted in their paper.
“Observable consequences of diminished alertness, such as attenuated response times and an increased frequency of errors, are well-documented behavioral markers of sleep loss.”