While the specter of cancer and Alzheimer’s disease looms large in the medical landscape, it’s a rare occurrence for an individual to be afflicted by both conditions simultaneously.
For a considerable period, epidemiological investigations have indicated an inverse correlation: individuals diagnosed with cancer appear to exhibit a diminished propensity for developing Alzheimer’s, and conversely, those with Alzheimer’s are less likely to contract cancer. However, the underlying rationale for this phenomenon has remained elusive.
A recent investigation conducted on murine subjects tentatively proposes a surprising hypothesis: certain neoplastic growths may actively disseminate a benign signaling molecule to the cerebral cortex, thereby facilitating the clearance of the deleterious protein aggregates implicated in Alzheimer’s pathology.
Alzheimer’s disease is fundamentally characterized by the accumulation of viscous amyloid beta protein deposits, which coalesce into extracellular plaques within the brain’s neural network. These proteinaceous deposits disrupt interneuronal communication and instigate inflammatory responses, ultimately leading to the progressive deterioration of cognitive functions and memory recall.
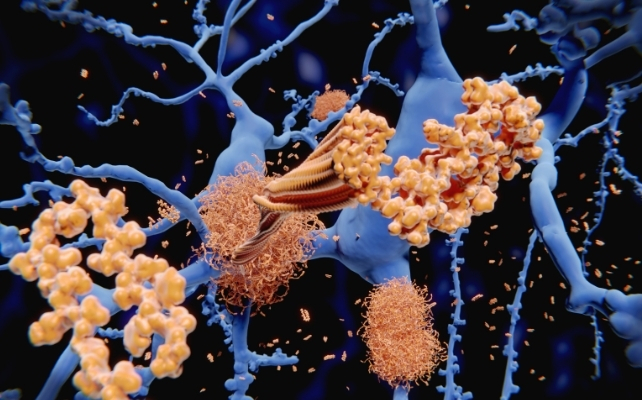
In the context of the novel research, scientists introduced human oncological specimens, specifically from lung, prostate, and colon tissues, beneath the epidermis of mice genetically predisposed to developing Alzheimer’s-like amyloid plaque formations. In the absence of intervention, these experimental animals invariably exhibit substantial amyloid beta accumulations in their brains as they mature, mirroring a cardinal characteristic of the human ailment.
However, the presence of these tumors markedly attenuated the accumulation of typical plaques within the rodents’ cerebral hemispheres. In certain experimental cohorts, a discernible enhancement in memory performance was observed when contrasted with Alzheimer’s model mice that did not harbor tumors. This finding suggests that the observed neuroprotective effect transcended mere microscopic alterations.
The research team identified a specific protein, termed cystatin-C, as the mediator of this effect. It was ascertained that the tumors were actively secreting this protein into the systemic circulation. The investigation posits that, at least within the murine model, cystatin-C released by tumors can traverse the blood-brain barrier—a highly selective physiological defense mechanism designed to safeguard the central nervous system from circulating substances.
Once within the brain parenchyma, cystatin-C appears to bind to nascent amyloid beta oligomers, thereby flagging them for degradation by the brain’s intrinsic immune operatives, known as microglia. These specialized cells function as the brain’s cellular sanitation brigade, perpetually surveying for cellular debris and improperly conformed proteins.
A characteristic of Alzheimer’s disease is the apparent impairment of microglial clearance mechanisms, permitting amyloid beta to aggregate and solidify into hardened plaques. In the tumor-bearing mice, cystatin-C was observed to activate a specific receptor on microglia, designated Trem2, effectively augmenting their phagocytic capacity and promoting aggressive plaque removal.
Intriguing Biological Trade-offs
At first apprehension, the notion that a malignant neoplasm could actively contribute to cerebral protection against dementia might seem counterintuitive, even paradoxical. Nonetheless, biological systems frequently operate via complex trade-offs, wherein a process detrimental in one context may prove advantageous in another.
In this particular scenario, the tumor’s elaboration of cystatin-C could represent an incidental byproduct of its inherent biological processes that coincidentally confers a beneficial outcome for the brain’s management of protein misfolding incidents. This does not imply that oncogenesis is inherently beneficial; rather, it illuminates a biological pathway that may offer therapeutic potential when modulated through more controlled means.
This research aligns with a burgeoning corpus of scientific inquiry suggesting that the nexus between oncological processes and neurodegenerative disorders extends beyond mere statistical coincidence. Extensive epidemiological surveys have consistently reported a significantly lower incidence of cancer diagnoses among individuals affected by Alzheimer’s disease, and vice versa, even after controlling for demographic and health-related variables.
This observation has given rise to the concept of a biological equilibrium, wherein cellular mechanisms promoting survival and proliferation, as seen in cancerous development, might concurrently inhibit the pathways conducive to neural degeneration. The cystatin-C narrative introduces a tangible molecular mechanism to this theoretical framework.
It is imperative to acknowledge that this research has been conducted exclusively in murine models and not in human subjects, a distinction of considerable significance. While rodent models of Alzheimer’s effectively recapitulate certain facets of the disease, notably amyloid plaque deposition, they do not fully encompass the intricate complexity of human neurodegeneration.
Furthermore, it remains to be elucidated whether human malignancies in actual patients generate sufficient quantities of cystatin-C or transport it to the brain in a manner that yields clinically meaningful impacts on Alzheimer’s disease risk. Nevertheless, this groundbreaking discovery offers promising avenues for the development of future therapeutic interventions.

One potential therapeutic strategy involves the creation of pharmaceutical agents or interventions designed to replicate the beneficial activities of cystatin-C without the involvement of neoplastic tissue. This could entail the development of genetically engineered variants of the protein with enhanced affinity for amyloid beta, or the identification of molecules capable of activating the same microglial pathways to augment their cellular clearance capabilities.
This body of research also underscores the profound interconnectedness of physiological systems, even when disparate organ systems are involved. A tumor originating in the pulmonary or colonic tissues might appear geographically remote from the gradual accumulation of proteinaceous deposits within the brain; however, molecules secreted by such a tumor can circulate systemically, surmount protective biological barriers, and modulate neuronal activity.
For individuals currently managing cancer or providing care for someone with Alzheimer’s, the immediate clinical implications of this research may be limited. Nonetheless, the findings offer a more optimistic perspective: through meticulous investigation of even formidable diseases such as cancer, scientists can uncover unforeseen insights that illuminate novel strategies for preserving cerebral vitality throughout the aging process.
Perhaps the most compelling takeaway is that the body’s protective mechanisms and its susceptibilities are seldom straightforward. A protein implicated in pathological processes within one organ may function as a crucial cleansing agent in another. By deciphering these intricate biological maneuvers, researchers may be able to harness them in a safe and effective manner to safeguard the aging human brain.
![]()