A collaborative effort from researchers at the Korea Advanced Institute of Science and Technology has yielded significant insights into the functionality of the PPARγ protein, a pivotal orchestrator responsible for the process of adipogenesis, or the genesis of fat cells.
The activation of PPARγ initiates a cascade of genetic expressions that steer a cell towards differentiation into, and subsequent maintenance as, a mature adipocyte.
Through meticulous analyses conducted on murine cells and established animal models, the scientific team has elucidated that the directive signals for adipogenesis mediated by PPARγ can be effectively stifled by a distinctive epigenetic mechanism—a switch that modulates gene activity without necessitating alterations to the underlying DNA sequence.
“This investigation represents the inaugural evidence demonstrating that adipocyte differentiation is governed with remarkable precision at the epigenetic stratum, extending beyond mere transcriptional regulation,” stated molecular biologist Dae-Sik Lim, as reported through a relevant publication.
Central to these findings are two key proteins, YAP and TAZ, which function as integral components of the Hippo signaling pathway. This pathway plays a fundamental role in the regulation of organ growth by dictating cellular decisions regarding proliferation, programmed cell death, or terminal differentiation into specific lineages, inclusive of adipocytes.
Prior scientific understanding alluded to an indirect influence of YAP and TAZ on adipocyte development, but the precise molecular underpinnings remained elusive. The present research endeavors to unpack these intricate biological mechanisms, offering a granular understanding akin to dissecting the precise operation of an engine.
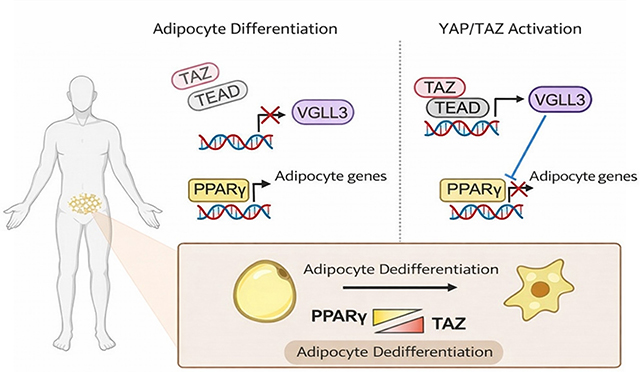
“Our comprehensive genomic investigations offer profound mechanistic insights into how the Hippo-YAP/TAZ axis dictates metabolic cell fate through the mechanism of epigenetic reprogramming,” the research authors articulated within their peer-reviewed publication.
Evidence presented indicates that YAP and TAZ facilitate a biochemical cascade that effectively deactivates the gene targets essential for adipocyte activation, which PPARγ endeavors to engage.
Consequently, while PPARγ may propel a cell towards adipocytic differentiation, YAP and TAZ possess the capacity to negate this directive, thereby maintaining the cell in a less differentiated state. The activity of YAP and TAZ is typically subject to stringent regulation by the Hippo pathway, which functions analogously to a braking system.
The investigators conducted experiments to observe the effects of inhibiting the Hippo signaling pathway in murine subjects, thereby disengaging the regulatory control over YAP and TAZ. In this condition of released inhibition, YAP and TAZ exhibited hyperactive states, leading to a de-differentiation of existing adipocytes.
These adipocytes did not revert entirely to a pluripotent stem cell state; rather, they relinquished many of their characteristic adipocytic attributes and assumed characteristics more closely aligned with those of precursor cells.
The culmination of this research provides a significantly enhanced comprehension of the mechanisms governing the augmentation or reduction of adipocyte populations, though currently limited to observations in animal models.
An overabundance of adipose tissue, or its ectopic deposition, is strongly correlated with a diverse array of health complications. Furthermore, established adipocytes are notably recalcitrant to elimination; during weight reduction, these cells typically diminish in volume rather than undergoing apoptosis, effectively persisting.
Elucidating the precise mechanisms by which PPARγ promotes adipogenesis, or fails to do so, could potentially pave the way for novel therapeutic interventions for metabolic disorders. The insights garnered from these discoveries may offer avenues for more targeted approaches to managing adipose tissue accumulation, though substantial further investigation is imperative to establish safe and efficacious clinical applications.
“This study has furnished a critical foundation for a more sophisticated grasp of the underlying mechanisms driving adipocyte identity modulation and, in the long trajectory, for the development of individualized therapeutic strategies for patients afflicted with metabolic diseases,” Lim concluded.