The widespread propagation of genes conferring antibiotic resistance represents a paramount and worldwide peril to public well-being. A newly published, extensive examination by researchers affiliated with Hohai University delves into the evolutionary genesis, the ecological factors driving the multiplication and spread of antibiotic resistance genes, and their profound environmental consequences.
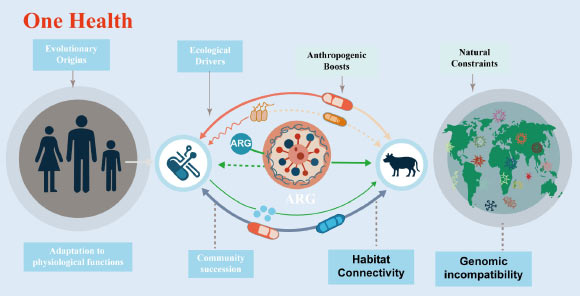
The evolutionary trajectory of genes responsible for antibiotic resistance is intrinsically tied to fundamental physiological functions and distinct ecological divisions. Image attribution: Xu et al., doi: 10.48130/biocontam-0025-0014.
The proliferation of antibiotic resistance genes has emerged as a chief global threat to public health, with their dissemination traversing the interconnected spheres of human, animal, and environmental systems.
These genes have been identified even in the most extreme and untamed environments on our planet, including the abyssal depths of the Mariana Trench, untouched tracts of Alaskan soil, and permafrost sediments dating back 30,000 years – settings completely devoid of any human-induced antibiotic exposure.
This pervasive distribution substantiates a critical reality: the capacity for bacterial species to withstand antibiotics evolved millions of years prior to the advent of antibiotics for medicinal and agricultural utilization.
“The phenomenon of antibiotic resistance did not commence with the advent of modern medical practices,” stated Dr. Guoxiang You, the senior author of the research.
“Many of the genes conferring resistance originally developed to aid bacteria in surviving environmental adversities, long before humanity’s discovery of antibiotics.”
“The genuine hazard today stems from the way human endeavors are dismantling natural barriers, thereby facilitating the transmission of these genes into pathogenic organisms.”
“A significant proportion of resistance genes originate from common bacterial genes that serve vital physiological purposes, such as the expulsion of noxious substances or the uptake of nutrients,” the investigators commented.
“Through the process of evolution, these genes acquired the capability to provide defense against antibiotics as a secondary function.”
Within natural, undisturbed ecological settings such as soils, freshwater bodies, and remote locales, the majority of resistance genes remain sequestered within specific microbial populations and present minimal risk to human health.
“A primary factor contributing to this localization is genomic incompatibility,” they elaborated.
“Bacteria that exhibit substantial genetic divergence often face difficulties in readily exchanging and operationalizing resistance genes.”
“This inherent biological mismatch functions as a protective barrier, curtailing the spread of resistance across disparate species and habitats.”
“Nevertheless, human activities are progressively weakening this protective mechanism.”
Within their review, the authors underscore how agricultural practices, effluent discharges from wastewater facilities, urban expansion, and global commerce are enhancing the interconnectedness of environments that were previously isolated.
The widespread use of antibiotics in both human medicine and animal husbandry imposes significant selective pressures, while the application of animal waste, the reuse of treated wastewater, and general environmental pollution converge bacteria originating from soil, animal, and human sources.
These amalgamated conditions create a more conducive environment for resistance genes to transfer into microbes that cause disease.
“Alterations in habitat connectivity driven by human actions fundamentally change the landscape,” remarked Dr. Yi Xu, the lead author of the scientific paper.
“When bacteria from diverse environments are repeatedly brought into close proximity under conditions of antibiotic pressure, resistance genes that were once innocuous can transform into a considerable threat to public health.”
“Agricultural lands treated with manure can also function as conduits, facilitating the movement of resistance genes from livestock into environmental bacteria, which can ultimately re-enter the human population via food, water, or direct contact.”
Crucially, the scientists emphasize that not all resistance genes carry the same level of danger.
A high prevalence in the environment does not inherently signify a commensurate high risk.
Gaining a thorough understanding of which genes are horizontally transferable, compatible with human pathogens, and associated with disease is indispensable for effective surveillance and containment strategies.
The researchers advocate for strategies that adopt an ecosystem-centric perspective to address antibiotic resistance.
These recommendations include curtailing the non-essential use of antibiotics, enhancing the efficiency of wastewater treatment technologies, implementing more conscientious management of animal waste and biosolids, and preserving relatively unblemished ecosystems that serve as benchmarks for natural resistance profiles.
“Antibiotic resistance is far more than solely a medical challenge,” Dr. You commented.
“It is an ecological predicament fundamentally rooted in our interactions with the environment.”
“Safeguarding the efficacy of antibiotics for future generations necessitates the contemporary protection of ecological integrity.”
“By synthesizing insights from evolutionary biology, microbial ecology, and environmental science, a holistic ‘One Health’ framework presents the most viable pathway forward in confronting one of the most significant global health crises of our era.”
The examination was made publicly available online on December 5, 2025, within the esteemed journal Biocontaminant.
_____
Yi Xu et al. 2025. Evolutionary origins, ecological drivers, and environmental implications of antibiotic resistance genes proliferation and dissemination: a ‘One Health’ perspective. Biocontaminant 1: e014; doi: 10.48130/biocontam-0025-0014