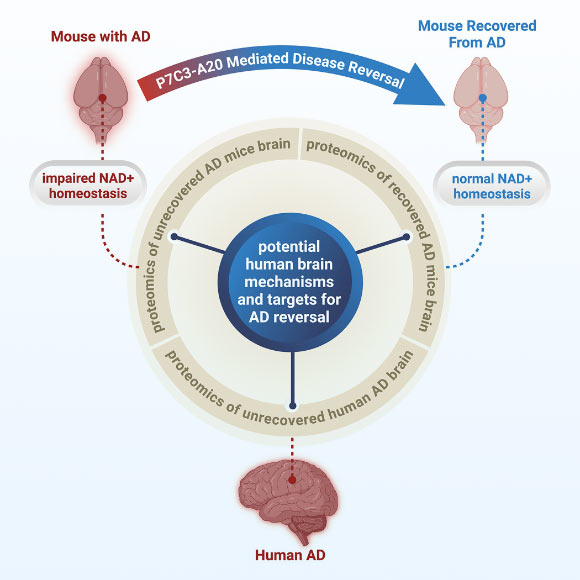
While traditionally regarded as an intractable condition, Alzheimer’s disease (AD) may be therapeutically reversible, according to a groundbreaking proof-of-principle study. A collaborative effort involving researchers from Case Western Reserve University, University Hospitals, and the Louis Stokes Cleveland VA Medical Center revealed that a critical cellular energy molecule, nicotinamide adenine dinucleotide (NAD+), plays a pivotal role. Their investigations, utilizing a range of preclinical mouse models and detailed examinations of human AD brains, confirmed that the impairment in maintaining normal NAD+ concentrations is a primary driver of AD pathogenesis. Furthermore, the research demonstrates that the restoration of appropriate NAD+ balance possesses the potential to both avert and ameliorate the disease.
Alzheimer’s disease, a condition recognized for over a century as irreversible, stands as the foremost cause of dementia. Projections indicate that by the year 2050, it is expected to impact more than 150 million individuals globally.
Contemporary therapeutic interventions, which focus on amyloid-beta (Aβ) clearance or management of clinical symptoms, offer only marginal benefits to patients, underscoring the urgent need for alternative and complementary treatment modalities.
Intriguingly, individuals who carry autosomal dominant AD mutations can remain asymptomatic for extended periods, even decades, prior to symptom manifestation. Moreover, some individuals, categorized as nondemented but exhibiting Alzheimer’s neuropathology, accumulate substantial amyloid plaques without experiencing cognitive decline.
These observations lend credence to the hypothesis of inherent brain resilience mechanisms that either retard or counteract disease progression. This suggests that the preservation or enhancement of such endogenous processes could offer a pathway to alter the disease trajectory or facilitate recovery from AD.
Maintaining NAD+ homeostasis is fundamental to cellular resilience against a spectrum of pathological insults, including oxidative stress, DNA damage, neuroinflammation, compromised blood-brain barrier integrity, diminished hippocampal neurogenesis, deficits in synaptic plasticity, and neurodegeneration.
In a recently published study, Professor Andrew Pieper from Case Western Reserve University and his research team demonstrated that a pronounced decline in NAD+ levels is a characteristic feature of human AD brains. This same phenomenon, they found, is replicated in murine models engineered to mimic the disease.
Although AD is a condition unique to humans, its study in laboratory settings is facilitated by the use of genetically modified mice that express human genetic mutations known to induce AD in people.
The research team employed two distinct mouse models: one incorporated multiple human mutations associated with amyloid processing, while the other carried a human mutation impacting the tau protein.
Both genetic lineages of these mice developed neuropathological hallmarks mirroring human AD. These included deterioration of the blood-brain barrier, axonal degeneration, neuroinflammatory responses, impaired hippocampal neurogenesis, reduced synaptic transmission efficiency, and widespread accumulation of oxidative damage.
Concurrently, these mice exhibited the characteristic severe cognitive impairments observed in individuals affected by AD.
Following the observation that NAD+ levels declined precipitously in both human and murine AD brains, the scientists proceeded to investigate whether preventing the loss of brain NAD+ balance prior to disease onset, or restoring it after significant disease progression, could prevent or reverse AD, respectively.
This investigation built upon their prior research, which had established that re-establishing the brain’s NAD+ balance led to both pathological and functional recovery following severe, long-standing traumatic brain injury.
The restoration of NAD+ balance was achieved through the administration of P7C3-A20, a pharmacologic agent that has been well-characterized for its efficacy.
Remarkably, not only did maintaining NAD+ balance shield the mice from developing AD, but even delayed treatment in mice with advanced disease allowed the brain to repair critical pathological events driven by the disease-causing genetic mutations.
Furthermore, both mouse cohorts experienced a complete recovery of cognitive function. This functional recovery was accompanied by normalized blood levels of phosphorylated tau 217, a recently validated clinical biomarker for AD in humans. This finding serves as confirmation of disease reversal and highlights a potential objective biomarker for future AD recovery clinical trials.
“Our findings were met with considerable excitement and encouragement,” stated Professor Pieper.
“The re-establishment of the brain’s energetic equilibrium resulted in both pathological and functional restoration in both lines of mice afflicted with advanced AD.”
“The observation of this effect across two disparate animal models, each driven by distinct genetic etiologies, bolsters the novel concept that recovery from advanced disease might be achievable in human AD patients through the restoration of the brain’s NAD+ balance.”
These findings are poised to instigate a fundamental shift in how researchers, clinicians, and patients approach the treatment of AD in the future.
“The paramount message conveyed by this research is one of optimism: the detrimental effects of Alzheimer’s disease may not be permanently etched,” Professor Pieper remarked.
“The compromised brain exhibits the capacity for self-repair and functional recovery under specific conditions.”
“Our study successfully demonstrated a drug-induced method for achieving this in animal models, and additionally identified candidate proteins within the human AD brain that may be pertinent to the potential for AD reversal,” added Dr. Kalyani Chaubey, a researcher at Case Western Reserve University and University Hospitals.
The pharmacological strategy employed in this research utilizes an agent (P7C3-A20) that empowers cells to maintain their appropriate NAD+ equilibrium even under conditions of overwhelming stress, without inducing supraphysiologic NAD+ elevations.
“This aspect is of significant importance when considering patient care, and clinicians should contemplate the possibility that therapeutic strategies focused on restoring brain energy balance could indeed present a viable pathway toward disease recovery,” Professor Pieper advised.
The results of this study have been published in the esteemed journal Cell Reports Medicine.
_____
Kalyani Chaubey et al. Pharmacologic reversal of advanced Alzheimer’s disease in mice and identification of potential therapeutic nodes in human brain. Cell Reports Medicine, published online December 22, 2025; doi: 10.1016/j.xcrm.2025.102535.