Investigating the neural pathways governing growth hormone secretion during slumber, neuroscientists affiliated with the University of California, Berkeley, and Stanford University have identified a novel regulatory feedback system within the brain responsible for maintaining precise equilibrium in growth hormone levels. This discovery holds potential for developing therapeutic interventions for individuals experiencing sleep disturbances linked to metabolic disorders such as diabetes, alongside neurodegenerative conditions including Parkinson’s and Alzheimer’s disease.
“While it’s generally recognized that growth hormone secretion is closely associated with sleep, this understanding has primarily stemmed from indirect blood measurements taken during sleep periods,” stated Dr. Xinlu Ding, a postdoctoral researcher at the University of California, Berkeley.
“Our research involves direct neural activity monitoring in mice to elucidate the underlying processes.”
“We are establishing a foundational neural circuit that can be leveraged for the development of diverse therapeutic approaches.”
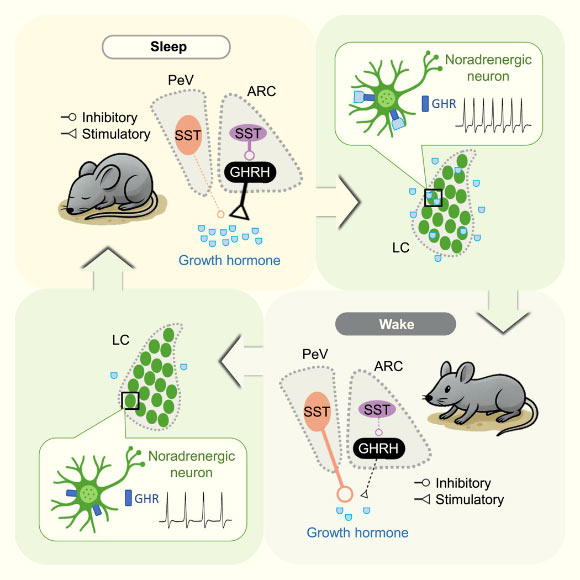
The specialized neurons that orchestrate the release of growth hormone in conjunction with the sleep-wake cycle—namely, growth hormone-releasing hormone (GHRH) neurons and two distinct types of somatostatin neurons—reside deep within the hypothalamus, a primordial brain region conserved across all mammalian species.
Upon its release, growth hormone amplifies the activity of neurons in the locus coeruleus, a brainstem region integral to arousal, attention, cognitive functions, and the exploration of novel stimuli.
Aberrant functioning of locus coeruleus neurons has been implicated in a multitude of psychiatric and neurological disorders.
“Gaining insight into the neural circuitry governing growth hormone release could ultimately pave the way for novel hormonal therapies aimed at enhancing sleep quality or rectifying imbalances in growth hormone levels,” commented Dr. Daniel Silverman, also from the University of California, Berkeley.
“There are emerging experimental gene therapy strategies that involve targeting specific cell populations.”
“This circuitry presents a novel avenue for modulating the excitability of the locus coeruleus, an approach that has not been previously explored.”
The researchers investigated this neuroendocrine circuit by implanting electrodes into the brains of mice and subsequently measuring neural activity fluctuations following light-induced stimulation of hypothalamic neurons.
Mice exhibit fragmented sleep patterns, with brief waking intervals lasting a few minutes interspersed throughout the day and night, thereby offering numerous opportunities to observe growth hormone dynamics across their sleep-wake cycles.
During REM sleep, somatostatin and GHRH surge to augment growth hormone secretion, whereas during non-REM sleep, somatostatin levels decline while GHRH increases only moderately, yet both contribute to enhanced growth hormone release.
The secreted growth hormone subsequently regulates the activity of the locus coeruleus, acting as a feedback mechanism to foster a homeostatic equilibrium akin to a yin-yang balance.
“This dynamic suggests that sleep and growth hormone operate within a finely tuned system: insufficient sleep leads to reduced growth hormone release, and conversely, excessive growth hormone can promote a state of wakefulness,” Dr. Silverman explained.
“Sleep initiates growth hormone release, and this hormone, in turn, feeds back to regulate wakefulness, with this delicate balance being paramount for growth, tissue repair, and metabolic well-being.”
Given that growth hormone exerts some of its influence via the locus coeruleus, which manages overall brain arousal during periods of wakefulness, achieving an appropriate balance could have far-reaching positive effects on attention and cognitive processes.
The investigation has been published in the esteemed scientific journal Cell.
_____
Xinlu Ding et al. 2025. Neuroendocrine circuit for sleep-dependent growth hormone release. Cell 188 (18): 4968-4979; doi: 10.1016/j.cell.2025.05.039