As individuals age, a multitude of physiological operations, including the capacity for tissue regeneration, tend to diminish in efficacy. A recent scientific investigation has illuminated a promising strategy to reinvigorate these crucial restorative processes.
This groundbreaking study, conducted by a collective of researchers at the University of California, San Francisco, pinpointed four transcription factors—proteins instrumental in regulating gene activity—that exhibit a revitalizing influence on cellular structures.
Upon augmenting the production of one of these identified transcription factors within the hepatic cells of aged murine subjects, the investigators observed a cascade of positive outcomes. These included a marked reduction in adipose tissue and fibrotic scarring, alongside enhanced glucose tolerance, all indicative of a more juvenile organ state.
The research cohort also manipulated the concentrations of all four transcription factors within human fibroblast cells cultivated in laboratory settings. Fibroblasts are integral to the formation of connective tissues, providing structural support to adjacent cells and organs. The adjustments made to these factors elicited multifaceted signs of cellular rejuvenation, such as increased proliferative capacity and elevated energetic levels.
“By modulating gene expression through the application of the transcription factors we’ve identified, aged fibroblasts demonstrated behavior characteristic of younger cells, thereby enhancing the vitality of elderly mice,” stated biochemist Hao Li.
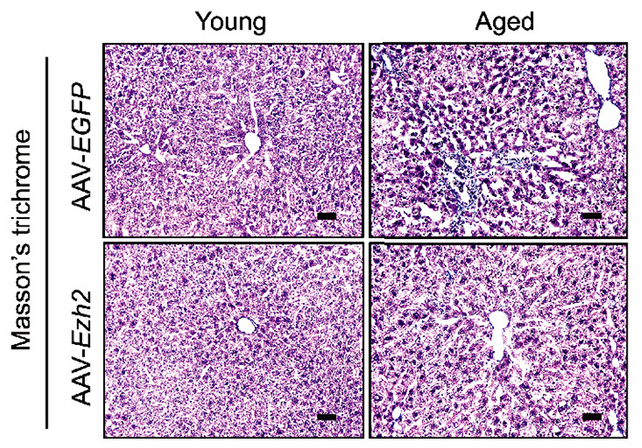
The process of identifying these four pivotal transcription factors commenced with a comparative analysis of aged versus young human fibroblast cells. This was achieved via a sophisticated computational model designed to delineate age-related divergences in gene expression patterns.
Following the compilation of a preliminary list encompassing 200 transcription factors potentially implicated in maintaining cellular ‘youthfulness’, the researchers embarked on an iterative process of activating and deactivating these factors, thus altering their respective production levels.
The outcomes derived from this experimental manipulation guided the researchers toward the identification of the definitive four transcription factors, which were subsequently subjected to more rigorous investigation: E2F3, EZH2, STAT3, and ZFX. Modifying the levels of these specific factors in both murine liver cells and cultured human fibroblasts resulted in a cellular shift towards a younger functional profile.
The observation that these protein factors exerted a discernible effect across two distinct species and varied cell types strongly suggests the potential existence of a universal biological mechanism. This mechanism could potentially be applicable for reactivating youthful cellular characteristics in aged tissues.
“These findings indicate the presence of a common set of molecular prerequisites for cellular and tissue rejuvenation that transcends species boundaries,” the researchers noted in their published findings.
It is essential to underscore that this research is still in its nascent stages. Current efforts are not directed towards life extension, regenerative medicine for limb replacement, or systemic bodily rejuvenation. The experimental data is presently limited to a select number of cell types.
Furthermore, rigorous evaluation of long-term safety considerations is imperative. The murine experiments were conducted over a limited timeframe of a few weeks, leaving potential long-term ramifications of this cellular rejuvenation approach unaddressed. It is noteworthy that excessive cellular proliferation associated with EZH2 has been implicated in the development of malignancies.
Nevertheless, in light of an aging global demographic and increasing lifespans, exploring avenues to enhance long-term bodily health warrants dedicated scientific inquiry.
“Our endeavors have unveiled compelling new avenues for comprehending, and ultimately mitigating, age-related maladies,” commented biochemist Janine Sengstack, highlighting the research’s significant implications.