With the progression of age, the immunological defenses of the body experience a gradual diminution in efficacy, rendering the organism more susceptible to pathologies. Researchers have unveiled a novel methodology for revitalizing a critical element of immune functionality, potentially enhancing well-being in advanced life stages.
A collaborative contingent from the Broad Institute of MIT and Harvard directed their investigations toward the thymus, a diminutive gland situated anterior to the heart, which plays an indispensable role in the maturation of T lymphocytes. These specialized immunological cells function as sentinels, discerning and combating adversarial agents such as neoplastic growths and infectious agents.
Commencing in early adulthood, the thymus undergoes involution and a reduction in its metabolic activity, thereby curtailing the generation of T cells. Within preclinical murine models, the scientific team successfully repurposed a segment of the hepatic tissue to serve as a thymic surrogate, effectively disseminating the molecular cues responsible for stimulating T-cell proliferation.
“As human beings advance in years, the immune system’s capacity begins to wane,” observes MIT neuroscientist Mirco Friedrich. “Our objective was to conceptualize strategies for sustaining this protective immunological barrier over an extended duration, which consequently prompted our consideration of methods to fortify immunity.”
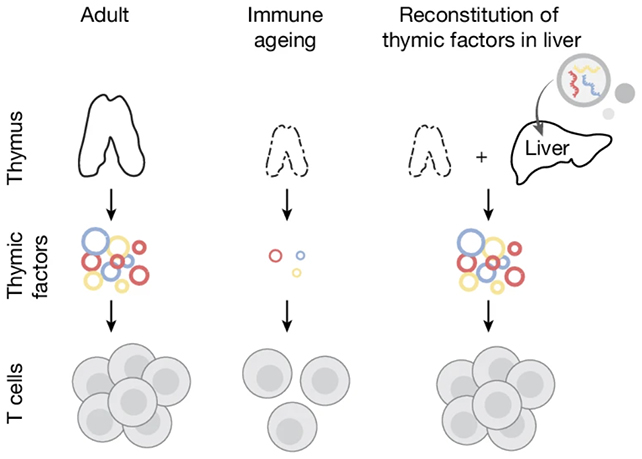
Initially, the investigative team conducted a comparative analysis between the immunological profiles of juvenile and aged rodents to pinpoint three paramount signaling proteins whose levels diminish with advancing age: DLL1, FLT3-L, and IL-7. These proteins are instrumental in the transformation of precursor cells into T cells and in maintaining their vitality.
Subsequently, an mRNA-based therapeutic formulation was meticulously prepared. Messenger RNA (mRNA), akin to a biological blueprint, provides the directives for protein synthesis. This therapeutic agent was administered intravenously on multiple occasions into the livers of elderly mice, thereby eliciting the intended signaling cascade.
The liver is intrinsically equipped for substantial protein synthesis, even in the twilight of an organism’s lifespan. Moreover, circulating blood originating from the gastrointestinal tract traverses the liver, rendering it an accessible organ for therapeutic interventions of this nature, thus establishing it as a highly suitable target.
Following a four-week regimen of the mRNA intervention in aged mice, significant enhancements were observed in both the quantity and heterogeneity of T cells present. These mice exhibited a more robust immunological response to inoculations and demonstrated a superior capacity to combat nascent tumor formations, indicative of a fortified, more youthful, and resilient immune system.
“Our methodology is characterized by its synthetic nature,” states MIT neuroscientist Feng Zhang. “We are essentially engineering the organism to replicate the secretory functions of the thymus.”
Crucially, the augmentation in T cell production achieved via the hepatic pathway was transient. This characteristic minimizes the potential for hyperstimulation of the immune system, a condition that could precipitate inflammatory responses and autoimmune reactions.
While the findings are encouraging, their applicability to human subjects requires validation beyond murine models. Future research endeavors by the scientists are slated to encompass the examination of alternative animal species, additional signaling molecules, and diverse immune cell populations.
Prior attempts at enhancing T cell production have been documented, encompassing the direct infusion of immunomodulatory agents into the circulatory system, which frequently carried attendant adverse effects and inherent risks. These nascent findings suggest that this liver-centric approach may present a safer and more efficacious alternative.
“Should we succeed in reinstating a fundamental physiological system like immunity, our aspiration is to facilitate individuals’ prolonged freedom from disease throughout their existence,” remarks Zhang.