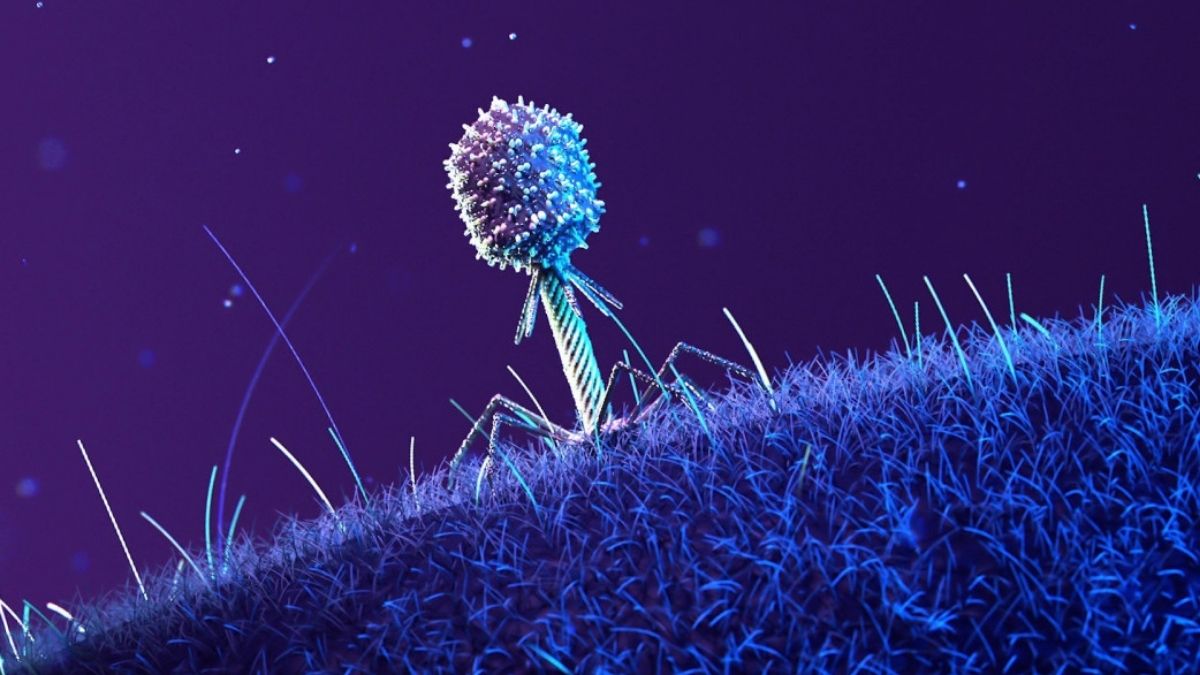
A container teeming with microbial life, encompassing viruses and bacteria, has successfully concluded its orbital expedition aboard the International Space Station. The transformations these microscopic organisms underwent during their extraterrestrial journey hold significant promise for humanity’s efforts to combat prevalent drug-resistant infections.
A collaborative scientific endeavor, involving researchers from the University of Wisconsin-Madison and the American biotechnology firm Rhodium Scientific Inc., orchestrated a confrontation between Escherichia coli bacteria and its primary viral adversary, the T7 bacteriophage. This biological pairing has been engaged in a protracted evolutionary struggle for an extensive period, but their interactions had never been observed under the conditions of microgravity until their dispatch to the ISS in the year 2020.
Stationed astronauts meticulously cultured various amalgamations of bacteria and phages over a span of 25 days. Concurrently, the investigative cadre, spearheaded by biochemist Vatsan Raman, replicated these identical experimental protocols at their terrestrial facility in Madison.
“The extraterrestrial environment profoundly alters the dynamics of phage-bacteria interactions, resulting in a deceleration of infectious processes and compelling both species to pursue divergent evolutionary paths compared to their terrestrial counterparts,” the scientists elaborate.
Within the weightless environment of space, the bacterial populations developed genetic alterations affecting their mechanisms for stress response and resource management. Furthermore, modifications were observed in their surface protein structures. Following an initial period of reduced activity, the phages responded by evolving genetic mutations that enabled them to maintain their capacity to attach to their hosts.
The research indicated that specific phage mutations, which arose in the space environment, demonstrated exceptional efficacy in eradicating the terrestrial strains of bacteria implicated in urinary tract infections (UTIs). It is noteworthy that over 90 percent of the bacteria responsible for UTIs exhibit resistance to antibiotics, positioning phage-based therapies as an exceptionally promising alternative.
“Through the meticulous examination of these space-induced adaptive changes, we unearthed novel biological understandings that permitted us to engineer phages exhibiting significantly augmented effectiveness against antibiotic-resistant pathogenic organisms on Earth,” the researchers state.