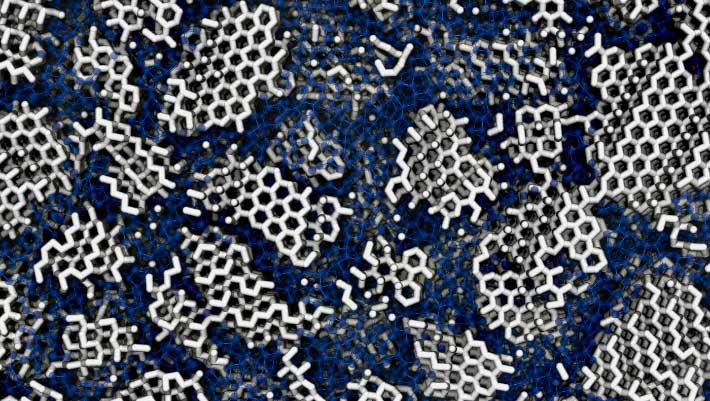
Despite its prevalence as one of the universe’s most common solid substances and its pivotal role in unraveling the perplexing anomalies associated with liquid water, the precise structural configuration of low-density amorphous ice remains a subject of scientific discourse, even nearly nine decades after its initial identification. A recent investigation undertaken by researchers affiliated with University College London and the University of Cambridge proposes that computational models simulating low-density amorphous ice align most closely with observed experimental data when the ice is understood not as entirely amorphous but as interspersed with minuscule crystalline regions, approximately 3 nanometers in width – a dimension marginally exceeding that of a single DNA strand. These tiny crystallites are purportedly embedded within the broader disordered matrix. Complementing this computational insight, the researchers also conducted experimental investigations involving the controlled recrystallization (thermal annealing) of actual amorphous ice samples prepared through diverse methodologies. Their findings revealed that the resultant crystalline architecture was contingent upon the genesis of the amorphous ice. The investigators concluded that if the ice had been wholly amorphous, exhibiting complete disorder, it would not have preserved any discernible structural imprints from its prior state.
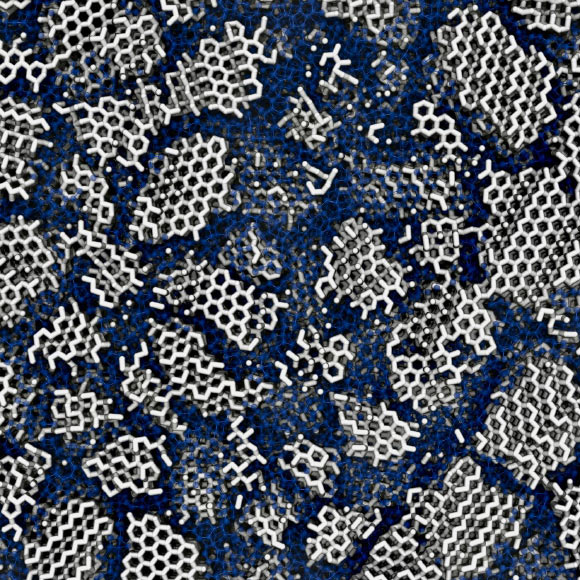
The structure of low-density amorphous ice: many tiny crystallites (white) are concealed in the amorphous material (blue). Image credit: Michael B. Davies, UCL & University of Cambridge.
“Our current understanding offers a detailed atomic-level depiction of the most abundant form of ice found throughout the cosmos,” remarked Dr. Michael Davies, a distinguished researcher from University College London and the University of Cambridge.
“This insight is of considerable importance given ice’s involvement in numerous cosmic phenomena, influencing aspects such as planetary formation, galactic evolution, and the distribution of matter across the universe.”
For the purposes of their study, Dr. Davies and his collaborators employed two distinct computational models simulating water.
These simulated ‘enclosures’ of water molecules were subjected to freezing by cooling to a temperature of minus 120 degrees Celsius (minus 184 degrees Fahrenheit) at varying rates.
The differing cooling velocities resulted in the formation of diverse proportions of crystalline and amorphous ice.
The research team ascertained that ice composed of up to 20% crystalline material (and consequently 80% amorphous) exhibited a structural profile that closely mirrored low-density amorphous ice, as evidenced by X-ray diffraction studies (a technique involving the analysis of X-ray scattering patterns).
Employing an alternative methodology, they constructed expansive ‘enclosures’ populated by numerous small ice crystals packed in close proximity.
Subsequent simulation of the disordered regions situated between these ice crystals yielded structural configurations remarkably similar to those obtained through the initial approach, which involved 25% crystalline ice.
In subsequent experimental endeavors, the scientists generated tangible specimens of low-density amorphous ice through a variety of preparation techniques. These ranged from the deposition of water vapor onto an exceptionally chilled substrate – simulating ice formation on cosmic dust particles within interstellar clouds – to the thermal processing of what is classified as high-density amorphous ice (ice subjected to immense pressure at cryogenic temperatures).
Subsequently, these amorphous ice samples were subjected to gentle warming, imparting sufficient energy for the crystallization process to commence.
Observable distinctions in the ice structures were noted, corresponding to their varied origins, particularly in the configuration of molecular stacking, specifically the prevalence of a six-fold (hexagonal) arrangement.
This observation served as indirect corroboration for the presence of crystalline structures within low-density amorphous ice.
The researchers reasoned that if the ice were entirely devoid of crystalline order, it would not retain any residual structural memory of its antecedent states.
These findings have instigated a cascade of additional inquiries concerning the fundamental nature of amorphous ices, including, for instance, whether the dimensions of embedded crystallites are influenced by the formation pathway of the amorphous ice, and indeed, whether a truly purely amorphous ice state is attainable.
“Water, the very essence of life, remains incompletely understood,” stated Professor Angelos Michaelides of the University of Cambridge.
“Amorphous ices may hold the key to elucidating some of water’s persistent anomalies.”
“In the extraterrestrial environment, ice possesses the potential to be a high-performance material,” Dr. Davies elaborated.
“It could offer protection to spacecraft from radiation or serve as a source of propellant, yielding hydrogen and oxygen.”
“Consequently, a comprehensive understanding of its diverse forms and properties is imperative.”
Furthermore, these discoveries carry significant implications for a speculative hypothesis regarding the origins of life on Earth.
This theory, known as Panspermia, postulates that the fundamental constituents of life were transported to our planet via comets composed of ice, with low-density amorphous ice conceptualized as the interstellar vehicle facilitating the conveyance of nascent organic molecules, such as simple amino acids.
“Our findings suggest that this type of ice would be a less effective medium for transporting these primordial molecules of life,” Dr. Davies commented.
“This is attributable to the fact that a partially crystalline structure offers reduced interstitial space for these components to become lodged.”
“However, the viability of the theory remains plausible, as amorphous regions persist within the ice where the building blocks of life could potentially find sanctuary and be preserved.”
“On Earth, ice’s nature is rendered a cosmic curiosity primarily due to our temperate climate,” observed Professor Christoph Salzmann from University College London.
“The inherent order of ice is readily apparent in the exquisite symmetry of a snowflake.”
“Conversely, ice elsewhere in the universe has long been posited as a frozen snapshot of liquid water, characterized by a disordered, fixed arrangement. Our research indicates this perspective is not entirely accurate.”
“Our conclusions also prompt contemplation regarding amorphous materials in broader technological contexts.”
“These materials are integral to numerous advanced technological applications.”
“For instance, the glass fibers employed for long-distance data transmission necessitate an amorphous, or disordered, state to fulfill their operational requirements.”
“Should these fibers contain latent microcrystals, their removal could lead to significant enhancements in performance.”
A scholarly article detailing these findings has been published today in the esteemed journal Physical Review B.
_____
Michael Benedict Davies et al. 2025. Low-density amorphous ice contains crystalline ice grains. Phys. Rev. B 112, 024203; doi: 10.1103/PhysRevB.112.024203