Cancer’s migration to new anatomical sites is facilitated by imperceptible vesicles. Elucidating the function of these nanoscale conveyances could revolutionize the battle against metastatic spread.
Our collective at the Department of Electrical Engineering within the École de technologie supérieure (ÉTS) is dedicated to impeding the systemic dissemination of malignant growths.
In concert with Prof. Julia Burnier and biological savants at the Research Institute of the McGill University Health Centre, our endeavors are focused on unraveling the mechanisms by which neoplasms transition into secondary tumors, or more precisely, how they infiltrate distant organs.
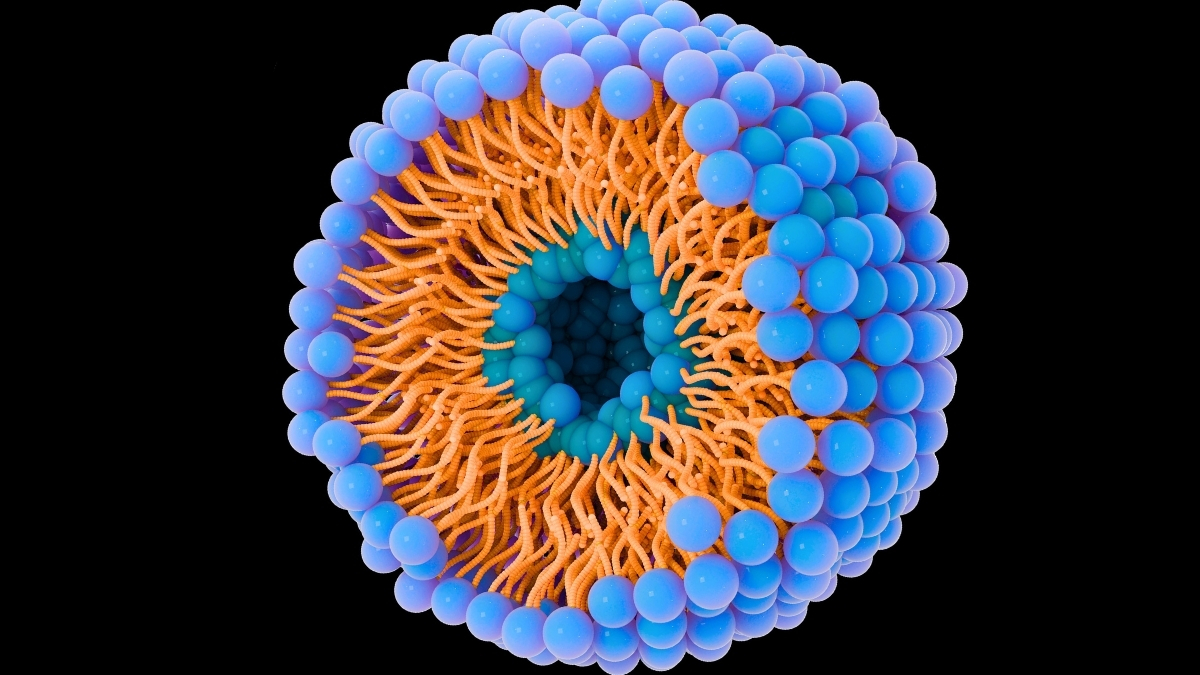
For approximately eight years, my research group has been immersed in the study of lipid nanoparticles, structures measuring a mere 100 nanometers, rendering them invisible to the unaided eye. Our inaugural objective involves comprehending the migratory pathways of cancerous cells. Subsequently, we endeavor to identify optimal strategies for therapeutic agent delivery into the organism.
Lipid nanoparticles, exemplified by liposomes, diverge from conventional oncological treatment modalities by facilitating the direct deposition of pharmaceuticals to neoplastic cells. This targeted delivery augments therapeutic efficacy while concurrently mitigating systemic toxicity compared to traditional cytotoxic chemotherapy.
Empirical evidence has emerged indicating that liposomes exhibit enhanced tumor targeting and a reduction in adverse effects. Furthermore, other investigations have documented that these nanomedicines improve treatment penetration and specificity, particularly in the context of metastatic disease.
These findings serve to corroborate the potential of nanomedicines to render cancer interventions more precise, more potent, and more easily tolerated.
Minute Particles Propel Dissemination
Every cellular entity within our physiology, be it healthy or malignant, extrudes minute particulate matter known as extracellular vesicles. These diminutive spheres, composed of lipids and proteins, also serve as carriers of genetic material.
When a cancerous cell expels its vesicles into the circulatory system and these are subsequently internalized by a healthy cell, they possess the capacity to modify the cell’s genetic code, thereby inducing oncogenic transformation. This phenomenon underlies the propagation of malignancy to other organs, such as the liver, and forms the basis of metastasis.
The principal impediment lies in the protracted and arduous nature of isolating and analyzing these endogenous vesicles. To accelerate our research trajectory, our team engineers synthetic analogues, termed liposomes, utilizing compact apparatuses known as micromixers.
Through the precise amalgamation of diverse constituents – lipids, proteins, aqueous solutions, and ethanol – our research cadre fabricates particles that closely emulate natural vesicles. The subsequent challenge involves discerning the specific lipid and protein complement within extracellular vesicles to facilitate the accurate synthesis of liposomes.
We then introduce these manufactured liposomes into hepatic carcinoma cells to meticulously observe their responses. The degree to which these cells retain the introduced particles serves as a robust indicator of the fidelity of our synthetic replicas.
In standard experimental protocols, liposomes are meticulously crafted with stringent parameters to precisely match the dimensions and electrostatic charge of endogenous extracellular vesicles. Furthermore, their visibility is enhanced through supravital fluorescent staining.
These meticulously prepared liposomes are subsequently incubated with neoplastic cells cultured within our laboratory milieu. This methodological approach facilitates real-time videography and quantitative measurement, unobtrusively, of the rate and manner of liposome internalization and subsequent expression by the cancerous cells.
Our observational data indicate a direct correlation: the greater the similitude in size and charge between liposomes and native vesicles, the more efficient their cellular uptake. This insight elucidates how their physicochemical attributes influence cellular assimilation and their potential implication in neoplastic proliferation.
Facialization of Liposomal Behavior
Our overarching objective is to elucidate the transport mechanisms of these extracellular vesicles to hepatocytes, thereby enabling the formation of secondary tumors. A paramount consideration is ensuring that these liposomes can authentically recapitulate the characteristics of extracellular vesicles.
Presently, we are achieving a protein encapsulation efficiency of approximately 50 percent. Our ambitious target is to elevate this to 90 percent. We anticipate that this enhancement will equip us to decipher the etiopathology of metastasis, thereby enabling its interception. Upon the refinement of this methodology, our team will proceed with preclinical investigations utilizing rodent models.
In the long-term panorama, this investigative thrust holds the potential to fundamentally alter patient outcomes by preempting the genesis of metastases and augmenting survival probabilities. Our ultimate aim: to unequivocally comprehend and effectively neutralize metastatic processes.
Pioneering Novel Therapeutic Avenues
Beyond merely dissecting the underlying mechanisms, our team is committed to forging innovative armamentaria against carcinoma. The conceptual framework involves leveraging these liposomes as microscopic delivery vehicles for targeted pharmaceutical agents directly to malignant cells.
The requisite diameters of these liposomes are calibrated based on the specific cancerous organ targeted for therapeutic intervention. Consequently, meticulous characterization and a profound understanding of liposomal properties are of utmost importance.
For instance, current research is investigating the encapsulation of curcuma, a compound extensively studied for its anticarcinogenic attributes. Our group is pursuing parallel investigations to ascertain the cellular responses to these curcuma-loaded liposomes.
Numerous studies have substantiated its anti-inflammatory and antioxidant contributions, which can potentiate the therapeutic effects of oncological treatments. By encapsulating curcuma within liposomes, we are significantly enhancing its capacity to reach and precisely target diseased cellular populations.
Unlocking the Enigmas of Cancer Proliferation
In addition to this natural compound, other therapeutic agents, such as paclitaxel, are already integrated into cancer treatment protocols in their liposomal formulation. The encapsulation of paclitaxel demonstrably improves drug delivery kinetics and enhances patient tolerance.
Furthermore, avant-garde strategies are employing liposomes for the conveyance of diminutive genetic fragments (DNA) or specialized proteins (antibodies) that function as intercellular signaling molecules, thereby augmenting the host’s immunological surveillance and combat against aberrant cells.
These advanced therapeutic modalities have undergone rigorous validation in multiple scientific investigations and are presently being deployed in select oncological treatment paradigms, with continuous annual advancements aimed at optimizing their efficacy and safety profiles.
By employing liposomes to meticulously mimic the endogenous vesicles released by cancerous cells, our research consortium endeavors to decipher the intricate secrets of cancer dissemination and delineate effective intervention strategies for its suppression. Our ongoing research is instrumental in paving the way for more precise therapies that can effectively prevent metastasis and improve patient prognoses.