A groundbreaking new investigation has revealed that limiting reproductive capabilities in certain mammalian species could potentially extend their lifespan by approximately 10 percent on average.
This research predominantly examines animals maintained in controlled environments such as zoological parks and aquatic centers globally. It has identified that numerous animal classifications, including primates, marsupials, and rodents, exhibit enhanced longevity following surgical sterilization or the implementation of contraceptive measures.
The degree of this life-extending effect varies among species, influenced by factors such as an animal’s sex, its environmental conditions, the timing of the intervention, and the specific procedure employed.
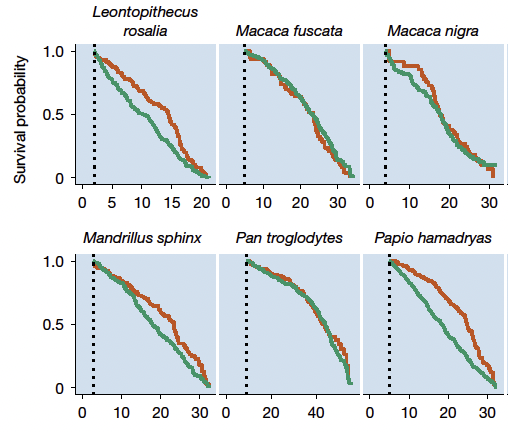
For illustration, female hamadryas baboons (Papio hamadryas) undergoing hormonal contraception demonstrated a life expectancy increase of 29 percent compared to their unsterilized counterparts, according to the study’s findings. Furthermore, male hamadryas baboons that were castrated experienced a 19 percent rise in longevity.
“This research underscores that the energetic demands associated with reproduction impose discernible and occasionally significant repercussions on survival rates across the mammalian class,” states Fernando Colchero, a specialist in statistical and mathematical ecology at the Max Planck Institute for Evolutionary Anthropology.
“A reduction in reproductive expenditure may permit a greater allocation of energy towards the maintenance of life.”
These observations lend support to a widely accepted evolutionary theory of aging, which posits a fundamental trade-off between the investment in reproduction and the resources allocated to DNA repair and somatic maintenance.
The prevailing hypothesis suggests that an organism possesses a finite energetic capacity throughout its existence. The act of producing offspring represents a substantial commitment, diverting a significant portion of this limited resource away from processes essential for growth and cellular regeneration.
Consequently, if an animal is precluded from reproducing due to the absence of necessary hormonal functions or anatomical structures, it may theoretically develop into a more robust and resilient individual.
To rigorously evaluate this proposition, Colchero and his colleagues meticulously analyzed the demographic records of 117 mammalian species with well-documented birth and mortality data, sourced from captive populations worldwide.
Additionally, the international research consortium conducted a comprehensive meta-analysis, synthesizing findings from 71 previously published studies focusing on sterilized animals. These studies encompassed a spectrum from highly controlled laboratory investigations to observational research conducted in natural habitats, with publication dates spanning from 1930 to 2021.
“The examination of zoological records offers an unparalleled perspective on the taxonomic diversity of lifespan responses, revealing that male castration, female surgical sterilization, and ongoing female hormonal contraception are associated with extended life expectancy across a broad array of species within the mammalian class,” the study’s authors assert.
Intriguingly, the lifespan-enhancing effects of sterilization were found to be comparable between male and female subjects. In male mammals housed in zoos, castration and analogous forms of irreversible surgical sterilization contributed to improved survival, whereas vasectomies did not yield similar benefits.
This finding suggests that a reduction in androgen levels might confer survival advantages in certain male animal populations, such as rodents, potentially by mitigating risky or aggressive behaviors.
Remarkably, the most substantial gains in longevity were documented in male mammals that underwent surgical sterilization at an early stage of development, even preceding puberty.
“This observation implies that the beneficial effect originates from the elimination of testosterone and its influence on fundamental aging pathways, particularly during early developmental periods. The greatest advantages are realized when castration occurs early in life,” elaborates lead author Mike Garratt from the University of Otago in New Zealand.
In contrast, among female mammals, various sterilization modalities were correlated with increased lifespans and a reduced incidence of infections. This could be attributed to these methods lessening the physiological burdens associated with gestation, lactation, and the cyclical reproductive processes – all of which consume energy that could otherwise be channeled into growth, repair, or immune system fortification.
Unlike their male counterparts in captive settings, the age at which sterilization occurred did not appear to significantly impact longevity in females (although the supporting data for this specific correlation was notably less robust than for males).
“This supports the theoretical advantages of menopause from an evolutionary standpoint, where a diminished investment in reproduction during later life contributes to enhanced longevity, thereby conferring fitness benefits through kin selection,” the study authors contend.
Cetaceans, for instance, are among the rare animal species, apart from humans, that undergo menopause, and they are known for their exceptionally long lives.
However, an extended lifespan does not invariably equate to an increased number of healthy years.
While sterilized female rodents may live longer, the researchers in the current study discovered that their subsequent health could be compromised. This phenomenon, termed a “health-survival paradox,” is also recognized in post-menopausal human females, who tend to outlive men but often experience heightened frailty and poorer overall health.
Extrapolating the implications of these findings to human populations presents considerable challenges due to data limitations. Historical records suggest that castrated men lived, on average, 18 percent longer, although the reliability of such historical accounts is a subject of debate.
For women, contemporary data pertaining to hysterectomies (surgical removal of the uterus) and oophorectomies (surgical excision of one or both ovaries) indicate a minimal effect size, potentially even in the opposite direction.
The meta-analysis revealed a one percent decrease in survival among women who had undergone these surgical procedures for non-malignant conditions.
“Reproduction is inherently resource-intensive,” explain Colchero, Garratt, and their colleagues. “However, human environments – through advancements in healthcare, nutrition, and social support systems – can mitigate or modify these inherent costs.”
The controlled environment of a zoological park provides an exceptional vantage point for observing evolutionary mechanisms that might otherwise remain obscured in natural settings.
This research was published in the esteemed scientific journal Nature.