As the human lifespan extends, subtle alterations in our DNA’s chemical landscape progressively emerge. Recent investigative work has unveiled that this phenomenon, termed ‘drift’ within intestinal stem cells, is propelled by inflammatory processes and compromised cellular communication pathways, potentially elucidating the escalating incidence of colorectal cancer with advancing age.
An international consortium of scientists has coined this cellular phenomenon Aging and Colon Cancer-Associated (ACCA) drift. It encompasses modifications in DNA methylation—a form of epigenetic alteration that dictates gene expression by adding or removing chemical tags without changing the underlying DNA sequence, thereby effectively activating or deactivating genes.
This specific drift mechanism results in the progressive inactivation of genes that play a critical role in tumor suppression, thereby fostering an accumulating risk of carcinogenic development across a growing number of intestinal cells, well in advance of any observable tumorous formations.
“We are witnessing an epigenetic signature that becomes increasingly pronounced as organisms age,” remarks Francesco Neri, a specialist in molecular biology affiliated with the University of Turin, Italy.
Building upon established knowledge—namely, the documented correlation between epigenetic drift and oncogenesis, and the age-dependent increase in colorectal cancer prevalence—the research team meticulously examined intestinal tissue samples from both healthy human colons and excised colon tumors. Their objective was to identify recurring methylation patterns.
The investigation revealed congruent patterns of gene silencing in elderly individuals and within cancerous tissues, strongly suggesting a shared underlying biological mechanism.
Subsequent investigations employing rodent models and sophisticated organoid cultures (miniature intestinal constructs cultivated in vitro) were instrumental in delineating the drivers of this drift and its propagation. These studies further confirmed the intestinal-specific nature of this process.
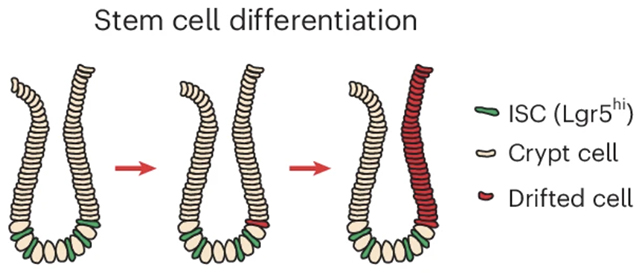
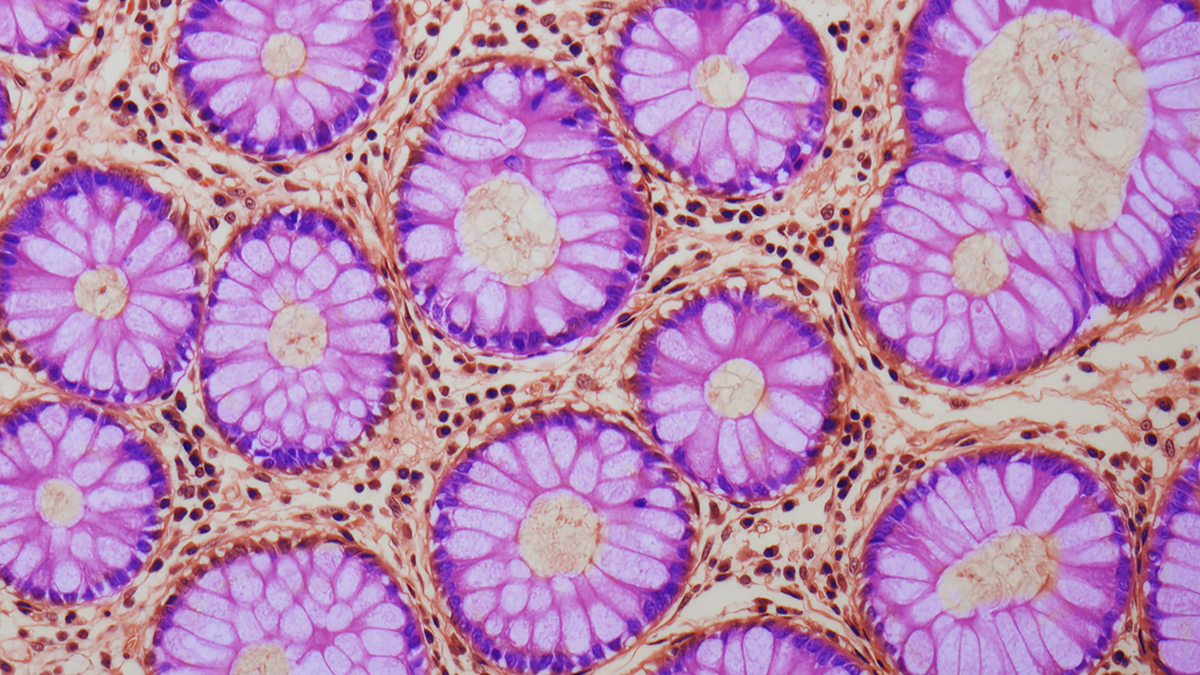
The research spotlighted intestinal crypts, the diminutive invaginations within the gut epithelium that harbor stem cells responsible for the continuous renewal of the intestinal lining. Experimental data indicated that ACCA drift initiates within these specialized stem cells and subsequently expands its reach concurrently with crypt proliferation and spread.
The underlying cascade involves a confluence of factors within intestinal crypt stem cells: heightened inflammation, diminished growth signaling, and reduced iron availability. These conditions collectively disrupt the cellular machinery responsible for maintaining methylation homeostasis, leading to the deactivation of crucial genes and thereby creating an environment conducive to malignant transformation.
“Over time, an increasing proportion of the tissue exhibits an aged epigenetic profile,” explains Anna Krepelova, a molecular biologist also based at the University of Turin. “Through the natural process of crypt division, these affected regions progressively enlarge and can continue their expansion over extended periods.”
“A deficit in cellular iron levels results in persistent aberrant modifications on the DNA. Consequently, the cells forfeit their capacity to rectify these epigenetic marks.”
As stem cell-driven crypts undergo division and multiplication, distinct areas of tissue characterized by older, cancer-susceptible epigenetic signatures gradually proliferate. This phenomenon leads to the formation of an increasing number of compromised zones throughout the intestinal tract over time.
The interplay of inflammation, iron dysregulation, and reduced proliferative signaling can collectively accelerate epigenetic drift. This implies that the aging process and the heightened susceptibility to oncogenesis might manifest earlier within the intestinal environment than previously hypothesized.
The distribution and extent of these high-risk zones are likely to exhibit inter-individual variability, mirroring the differences in cancer risk observed among people. However, this research provides novel insights into the mechanisms by which colorectal cancer gains a greater foothold as individuals age.
Encouragingly, laboratory experiments conducted on organoids demonstrated the feasibility of attenuating and, in some instances, partially reversing epigenetic drift. This was achieved through interventions aimed at augmenting iron uptake or reinstating specific cellular growth signals.
“This finding suggests that epigenetic aging is not necessarily an immutable, terminal state,” states Krepelova. “For the first time, we have evidence indicating that it is possible to modulate the fundamental parameters of cellular aging at a deep molecular level.”