A key enzyme instrumental in modulating systemic inflammation has been identified as a principal regulator of genes implicated in neurodegenerative processes, presenting substantial implications for Alzheimer’s disease and the aging of the brain.
Investigations conducted by a collaborative team from the University of New Mexico and the University of Tennessee involved a series of experimental assays on human tissue cultures. These studies focused on the consequences of inhibiting the activity of an enzyme designated as OTULIN.
Upon suppression of OTULIN’s enzymatic function within cellular environments, the research team observed a significant decrease in the abundance of tau, a protein strongly correlated with Alzheimer’s disease. Furthermore, complete ablation of the gene responsible for OTULIN production resulted in the complete cessation of tau synthesis.
Remarkably, this elimination of tau did not appear to compromise the viability or functional integrity of the neuronal cells.
A comparative analysis was performed between neurons sourced from an Alzheimer’s patient and neurons derived from stem cells of healthy individuals. This comparison revealed a higher prevalence of both OTULIN and tau in neurons exhibiting characteristics of the disease.
“Pathological tau is recognized as a primary determinant in both the aging of the brain and the development of neurodegenerative conditions,” stated molecular geneticist Karthikeyan Tangavelou of the University of New Mexico. he noted.
“By impeding tau biosynthesis through the targeted inhibition of OTULIN in neurons, it may be possible to reinstate cerebral health and mitigate the effects of brain aging.”
The prospect of utilizing OTULIN disruption or elimination as a therapeutic strategy to decelerate brain aging is currently considered impractical, at least in the near term. This is owing to the fundamental roles played by both the enzyme and tau in essential bodily functions.
As highlighted by the research cohort, any intervention involving OTULIN modulation would necessitate meticulous oversight to avert unintended deleterious effects in other physiological systems.
“Our investigation elucidated OTULIN’s specific function within neurons,” explained Tangavelou. “Our understanding of how OTULIN operates in other neural cell populations remains limited.”
Notwithstanding these limitations, the findings are both intriguing and unexpected, holding considerable promise for future scientific endeavors. One of the most promising avenues for combating Alzheimer’s disease appears to lie in the removal of the detrimental protein aggregates commonly associated with the condition. The present research introduces a novel pathway through which this might be achieved.
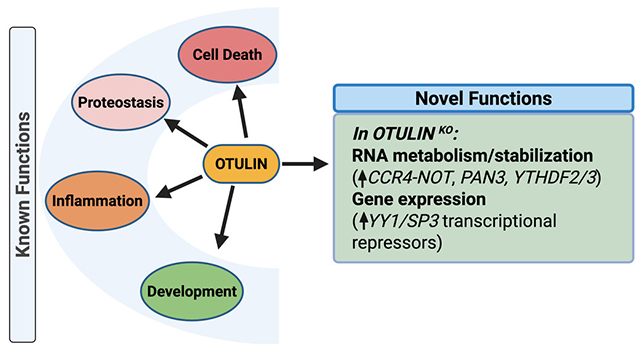
The research team extended their analysis by employing RNA sequencing to ascertain the broader impacts of OTULIN depletion. This revealed not only the cessation of tau production but also significant alterations in the expression of numerous other genes.
These affected genes were predominantly linked to inflammatory responses, suggesting that OTULIN plays a crucial role in managing neuronal stress and preventing cumulative damage within the brain under suboptimal operating conditions.
While these hypotheses necessitate validation through further investigation in animal and human models, scientists now potentially possess an additional therapeutic target for the development of interventions against Alzheimer’s and related neurological disorders. Moreover, OTULIN is not the sole enzyme currently under intense scientific scrutiny.
It is understood that one of OTULIN’s functions involves facilitating the cellular process of waste removal, which includes the clearance of protein aggregates such as tau. Malfunction of this enzymatic process can lead to the accumulation of cellular debris.
“This presents a significant opportunity to launch numerous research projects aimed at reversing brain aging and fostering a healthier brain,” concluded Tangavelou.