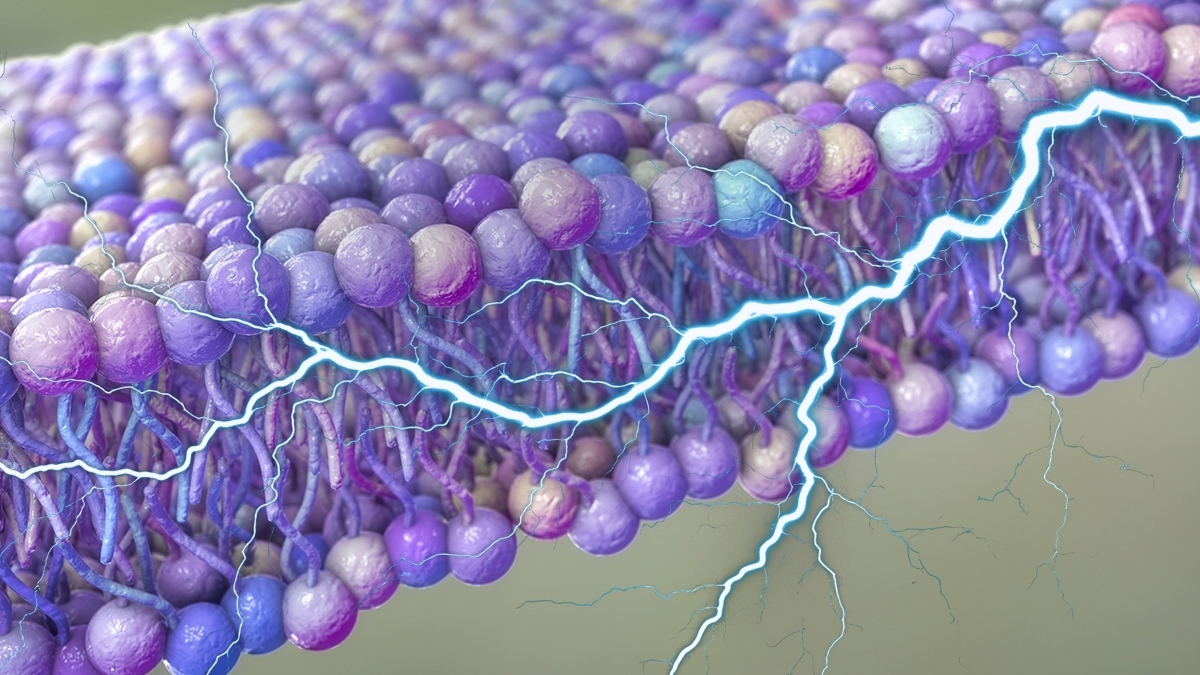
Our cellular structures might be intrinsically generating electrical impulses, functioning as an inherent energy reservoir that could facilitate material translocation or even participate in our organism’s intercellular signaling networks.
A collaborative research endeavor between the University of Houston and Rutgers University in the United States posits that subtle undulations within the lipid bilayers enveloping our cells could be capable of producing sufficient electrical potential to serve as a direct energy conduit for certain physiological processes.
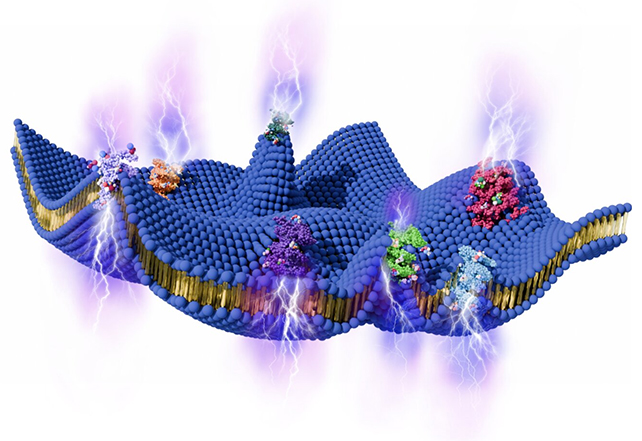
These inherent fluctuations have already been the subject of rigorous investigation, and it is understood that they are propelled by the operational dynamics of integral proteins and the metabolic breakdown of adenosine triphosphate (ATP), the ubiquitous cellular currency for energy transfer.
The contemporary investigation offers theoretical corroboration for the hypothesis that membrane oscillations possess the requisite amplitude and organizational coherence to establish an electric charge that cells can leverage for specific essential functions.
“Cells are not inert entities; they are animated by intrinsic active mechanisms, such as protein exertion and ATP catabolism,” state the investigators in their published scholarly work.
“We demonstrate that these dynamic fluctuations, when synergistically interacting with the pervasive electromechanical phenomenon of flexoelectricity, can engender transmembrane electrical potentials and even orchestrate ion flux.”
Central to a comprehensive grasp of this novel theoretical framework is the principle of flexoelectricity, which fundamentally elucidates the mechanism by which an electrical potential difference can arise across disparate points of mechanical deformation within a material.
Cellular membranes are in a perpetual state of conformational change due to the ceaseless thermal agitation inherent within the cellular environment. Theoretically, any electrical potential generated through this process should nullify itself in systems at equilibrium, thereby rendering it ineffective as an energy source.
The researchers posited that cellular environments are not in a state of strict thermodynamic equilibrium, given the continuous internal activities essential for sustaining life. The critical question was whether these ongoing processes were sufficiently robust to transform a lipid membrane into a functional power generator, necessitating several detailed quantitative analyses.
According to the computational models developed by the research team, flexoelectricity has the potential to establish an electrical gradient across the cell boundary, potentially reaching up to 90 millivolts – a magnitude sufficient to trigger neuronal depolarization.
The resultant voltage could significantly contribute to the translocation of ions, which are charged atomic species whose movement is governed by electrochemical gradients.
Membrane fluctuations may possess sufficient influence to modulate physiological operations, including neuromuscular contraction and the transmission of sensory information. The researchers estimated that these charge separations manifest on the millisecond timescale, aligning perfectly with the temporal dynamics of signals propagating through neural pathways.
“Our findings indicate that cellular activity can substantially augment transmembrane potential and polarization, thereby proposing a physical paradigm for energy capture and directed ion movement within living cells,” articulate the investigators.
These discoveries hold the potential for broader applicability, extending to multicellular ensembles, and could elucidate the mechanisms by which cellular membranes coordinate to produce macroscopic effects in tissues. Subsequent investigations are now warranted to experimentally validate these theoretical predictions within in vivo biological systems.
The implications of these findings may transcend the confines of biological tissues; the researchers propose the conceptualization of employing these very electricity-generating principles to guide the design of artificial intelligence architectures and synthetic biomimetic materials.
“The exploration of electromechanical dynamics within neural networks could serve as a nexus between molecular flexoelectricity and intricate information processing, yielding profound insights into neural function and fostering the discovery of bio-inspired computational substrates,” conclude the researchers.