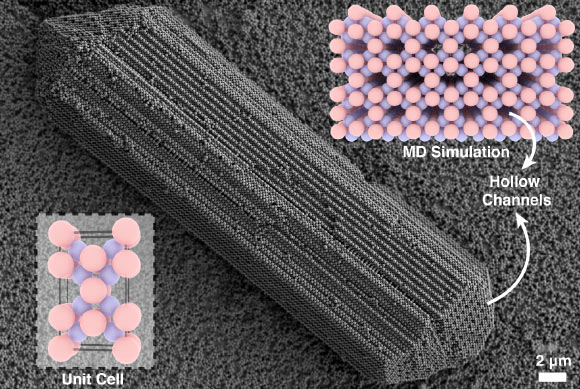
While investigating the mechanisms of crystal formation, a team of researchers affiliated with New York University encountered a novel, rod-shaped crystalline structure that had not been previously documented.
Crystals are defined as solid materials characterized by the arrangement of their constituent particles in recurring, ordered patterns.
This process, wherein order emerges from a state of disarray through self-assembly, was traditionally believed to adhere to a conventional, predictable growth paradigm.
However, it is now understood that crystal development can proceed via more intricate routes, deviating from the simple, sequential addition of constituent units.
To facilitate the study of crystalline material genesis, some researchers employ synthetic crystals composed of minuscule spherical entities known as colloidal particles. These particles, though diminutive, are considerably larger than the atoms that comprise other crystalline substances.
“The significant benefit of examining colloidal particles lies in our capacity to observe crystallization phenomena at the individual particle level. This is exceedingly challenging with atoms, given their minute size and rapid motion,” explained Professor Stefano Sacanna of New York University.
“Using colloids, we are able to visually track crystal formation under microscopic observation.”
In an effort to elucidate the formation pathways of colloidal crystals, Professor Sacanna and his collaborators conducted experiments designed to meticulously observe the behavior of charged colloidal particles under varied environmental and developmental conditions as they transitioned from saline suspensions to fully consolidated crystalline structures.
Concurrently, they executed numerous computer simulations to model the growth patterns of crystals, thereby aiding in the interpretation of their experimental findings.
Their analysis revealed that colloidal crystals precipitate through a bifurcated process: initial aggregations of particles form in a disordered state before undergoing a transformation into ordered crystalline architectures, leading to a wide spectrum of crystal classifications and morphologies.
During the course of these investigations, Shihao Zang, a doctoral candidate at New York University, identified a rod-like crystalline specimen that defied immediate classification.
While superficially resembling previously identified laboratory crystals to the unaided eye, closer scrutiny indicated a distinct particle composition and the presence of internal hollow conduits at its extremities.
A comparative analysis against an extensive compendium of over a thousand naturally occurring crystal structures yielded no correlative match.
By leveraging computational modeling, the research team generated a simulation of an identical crystal, which afforded them the opportunity to scrutinize its elongated, hollow morphology in enhanced detail.
“This observation was perplexing, as crystals are typically characterized by their density, yet this specimen featured void channels extending along its entire length,” commented Dr. Glen Hocky from New York University.
“Through this synergistic integration of experimental observation and computational modeling, we ascertained that this particular crystal architecture had never been previously documented,” Professor Sacanna elaborated.
The newly identified crystal was designated L3S4, referencing its elemental composition. However, it was informally referred to as ‘Zangenite’ during laboratory discussions, in recognition of Zang’s role in its discovery.
“Our study of colloidal crystals serves as a proxy for understanding real-world atomic crystals; however, we had not anticipated discovering a crystalline form that deviates from what is observed in nature,” Zang remarked.
The advent of Zangenite presents a novel avenue for exploring the potential applications of low-density, hollow crystalline structures and may facilitate the identification of further undiscovered crystalline entities.
“The internal channels within Zangenite share functional similarities with existing materials utilized for filtration or encapsulation purposes,” noted Dr. Hocky.
“Previously, we presumed that encountering novel crystal structures would be an infrequent occurrence, but it is now conceivable that we may uncover additional, as yet uncharacterized, structural arrangements,” Professor Sacanna stated.
A publication detailing this research has been released in the esteemed journal Nature Communications.
_____
S. Zang et al. 2025. Direct observation and control of non-classical crystallization pathways in binary colloidal systems. Nat Commun 16, 3645; doi: 10.1038/s41467-025-58959-0