Utilizing cryo-electron microscopy, scientific investigators have meticulously examined the architecture of Bas63, a bacteriophage belonging to the Ounavirinae subfamily and the Felixounavirus genus that specifically targets Escherichia coli, with the objective of elucidating its evolutionary connections and functional adaptations.
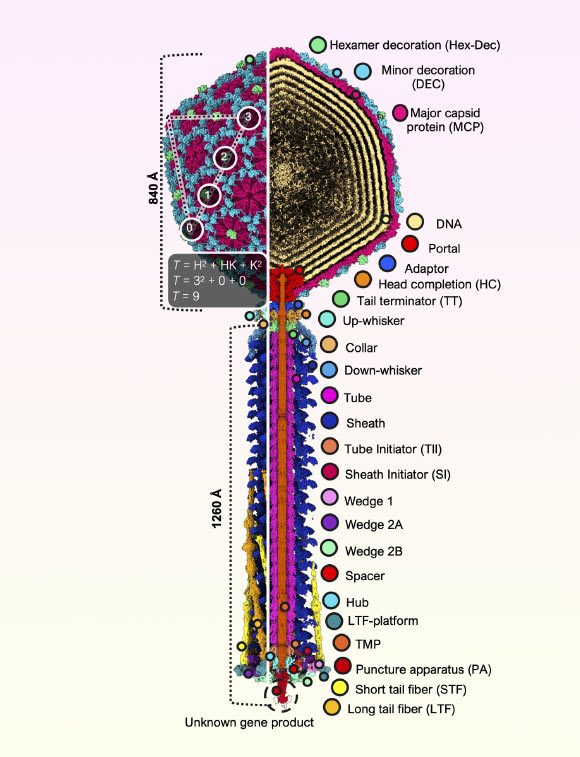
Composite representation of the complete Bas63 virion. Image credit: Hodgkinson-Bean et al., doi: 10.1126/sciadv.adx0790.
Viruses classified as bacteriophages within the Caudoviricetes class constitute the most prevalent viral group on our planet.
The Caudoviricetes encompass seven orders, seventy-four families, and one hundred and twenty-one subfamilies.
“Bacteriophages are a source of immense scientific fascination as the global research community actively seeks viable antibiotic alternatives to counteract the escalating threat of antimicrobial resistance,” remarked Dr. James Hodgkinson-Bean, a researcher affiliated with the University of Otago.
“Bacteriophage viruses pose no threat to any multicellular organisms and possess the remarkable ability to selectively target and eradicate specific bacteria.”
“Consequently, they are increasingly becoming the subject of extensive research and implementation in ‘phage therapy’ for the treatment of highly drug-resistant bacterial infections.”
“Bacteriophages are characterized by their exquisite and complex viral structures, which facilitate bacterial infection through sophisticated mechanical appendages referred to as ‘tails’.”
For the purposes of this investigation, Dr. Hodgkinson-Bean and his associates undertook a detailed molecular analysis of Bas63’s structure to gain a comprehensive understanding of its tail mechanism during the infection process.
“This line of inquiry is paramount for determining how to identify the most effective bacteriophages for therapeutic applications and for comprehending the variations in infectious behaviors observed in laboratory settings,” stated Dr. Hodgkinson-Bean.
“With the escalating prevalence of antibiotic resistance and the growing threat of plant pathogens to global food security, bacteriophages present a highly promising alternative solution,” added Dr. Mihnea Bostina, also a member of the University of Otago team.
“Our meticulously detailed structural blueprint of a bacteriophage represents a significant advancement in the rational design of strategies for medical, agricultural, and industrial applications, ranging from treating infections to mitigating biofilms in food processing facilities and water systems.”
“Beyond their scientific implications, the three-dimensional data—which elucidates the virus’s seldom-observed whisker-collar connections, hexamer decoration proteins, and varied tail fibers—holds the potential to inspire artists, animators, and educators.”
An in-depth comprehension of these viral structures also contributes to understanding their evolutionary trajectories.
“While DNA typically serves as the most reliable evolutionary marker in humans, the three-dimensional architecture of a virus offers more profound insights into its deep evolutionary relationships with other viral entities,” explained Dr. Hodgkinson-Bean.
The research team identified structural features that had previously only been observed in viruses with highly divergent evolutionary lineages, thereby revealing previously unrecognized evolutionary connections between them.
“Through structural investigations, we are aware that bacteriophages share evolutionary ties with herpesviruses—a relationship believed to extend back billions of years, predating the emergence of multicellular life,” Dr. Hodgkinson-Bean conveyed.
“For this reason, when we examine bacteriophage structures, we are essentially observing living fossils, ancient entities from primordial times.”
“There is an inherent and profound beauty in that realization.”
The discoveries were formally presented on November 12, 2025, within the esteemed pages of the journal Science Advances.
_____
James Hodgkinson-Bean et al. 2025. Cryo-EM structure of bacteriophage Bas63 reveals structural conservation and diversity in the Felixounavirus genus. Science Advances 11 (46); doi: 10.1126/sciadv.adx0790